Digital Autopsy Services
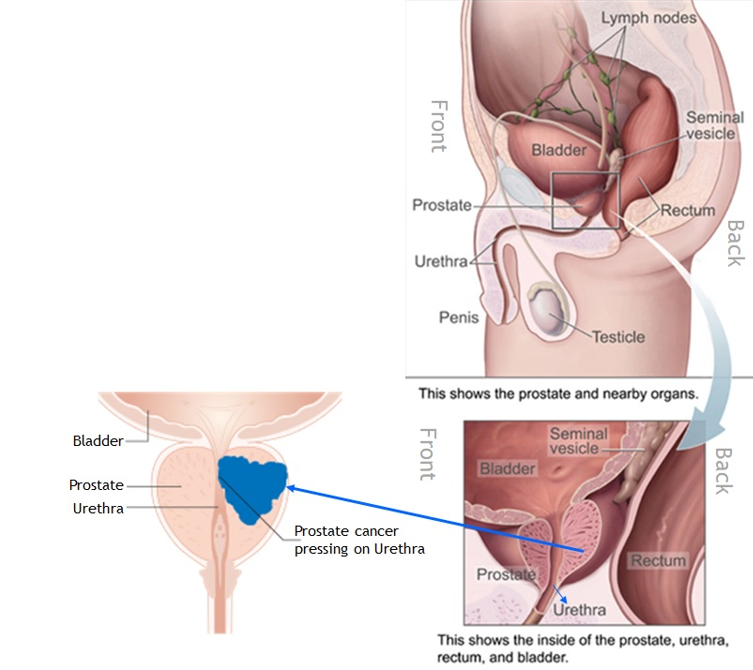
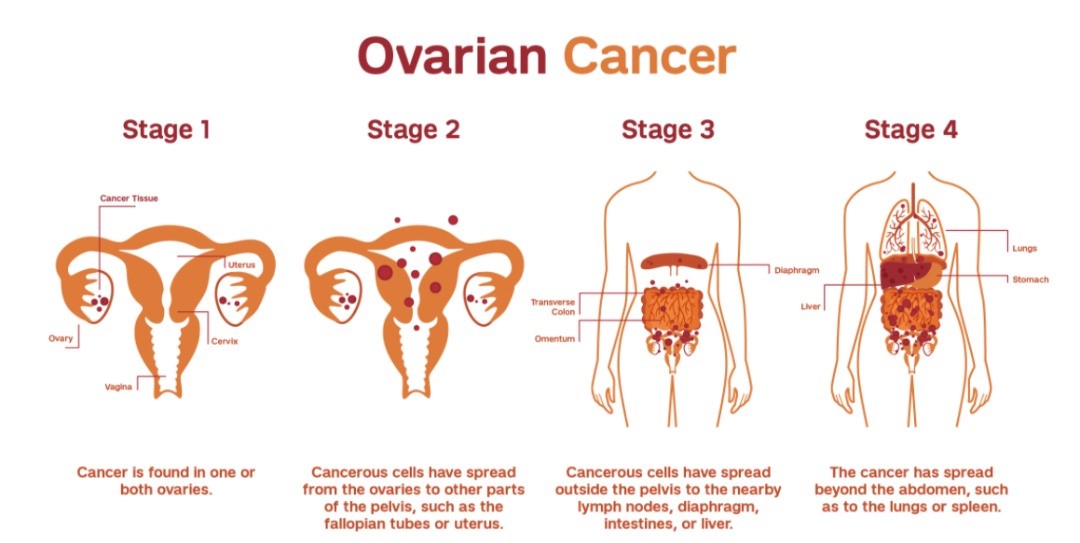
Experience a revolutionary transformation in forensic investigations with digital autopsy solutions. This cutting-edge technology redefines autopsy procedures, enabling detailed examination of the deceased without the need for invasive cuts or incisions. By leveraging advanced imaging techniques such as CT scans, forensic experts can access areas of the body that are often challenging or impossible to examine with traditional methods..
Digital autopsies provide a non-invasive, respectful approach, preserving the integrity of the body while offering unparalleled insights into the cause of death, injuries, or internal abnormalities. This innovative method enhances the precision and efficiency of forensic investigations and addresses cultural or religious sensitivities surrounding invasive procedures.
In addition to being a more dignified alternative, digital autopsies are faster, reduce the risk of contamination, and allow for the re-examination of findings without requiring additional physical procedures. Welcome the future of forensic science, where advanced technology empowers professionals to solve mysteries with precision, respect, and efficiency!